|
Veiranto, M (1), Tiainen, J (2), Niemelä, S (2),
Suokas, E (3), Ikäheimo, I (2), Koskela, M (2), Syrjälä,
H (2), Ashammakhi, N (1), Törmälä, P (1)
1 Tampere University of Technology, Tampere, Finland
2 University of Oulu, Oulu, Finland
3 Linvatec Biomaterials, Tampere, Finland
Introduction
Bone infection is a serious clinical complication that may follow surgery
or trauma. Besides general measures, and systemic prophylactic antibiotics,
a local drug-delivery system is thus needed. Our group has recently
developed the first reliable antibiotic-releasing miniscrews for bone
surgery in the world. Those screws are bioabsorbable and based on high
molecular weight self-reinforced (SR) poly(lactide-co-glycolide) (PLGA).
The aims of this study were to study in vitro drug release and mechanical
properties, bacterial inhibitory effect and biocompatibility of those
recently developed antibiotic releasing SR-PLGA miniscrews.
Materials and methods
The bioabsorbable matrix polymer in studied miniscrews was
semicrystalline poly(lactide-co-glycolide) (PLGA 80:20) (Purac) with intrinsic
viscosity
of 6.3 dl/g (0.1%, chloroform, 25°C). The antibiotic was ciprofloxacin.
It has a wide range of activity against osteomyelitis-causing bacteria
and good penetration to compact bone. PLGA and ciprofloxacin were extruded
into billets and then die-drawn into self-reinforced rods. Screws with
the same geometry as that of BioSorbPDXÒ 1.5 Screws (Linvatec Biomaterials,
Ltd.) and length of 4.0 mm were machined from self-reinforced rods. The
finished screws were gamma sterilized.
To determine the released ciprofloxacin concentration in vitro, screws
(500 mg) were placed into 50 ml phosphate buffer solution (KH2PO4 and NaOH)
at pH of 7.4. Five parallel samples were kept in an incubator shaker at
temperature of 37°C and at the specific sampling times, the released
drug concentrations were measured using UV-spectrometer (UNICAM UV 500)
at l=270.5 nm. Mechanical properties of the screws were determined using
an Instron 4411 materials testing machine. The shear strength of the screws
was determined using method modified from standard method ASTM B 769-87.
The torsion strength of the screws was measured by the test modified from
ASTM F 117-79. Five parallel samples were tested and means were calculated.
Biomechanical testing (pull out test) was also carried out using human
cadaver bones. Screws were tested for their bacterial inhibitory effect
by embedding in agar dishes containing ca. 107 cfu/ml S. epidermidis ATCC
35989 and inhibition areas were measured. Screws were also implanted in
cranial bone of rabbits to assess tissue reactions, biodegradation and
drug bone concentration.
Results
After 160 days in vitro all loaded ciprofloxacin
was released from the studied miniscrews (Fig.1). During that time
measured average
concentration
of released antibiotic per day was between 0.8 and 11.5 µg/ml
after the start-up burst peak. The maximum release occurred in the
8th week.
In vivo ciprofloxacin released from the studied miniscrews resulted
in achieving the highest bone tissue ciprofloxacin concentration of
14100
ng/g of bone at 4 weeks.
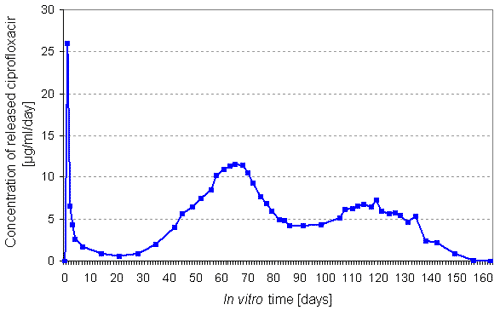
Fig.1. Concentration of released ciprofloxacin from studied bioabsorbable
gamma sterilized SR-PLGA miniscrews.
Initial mechanical properties of the studied miniscrews were high and
their application was easy. Initial shear strength of the studied miniscrews
was 172 MPa. Torsion strength of the screws was 65 MPa. Miniscrews retained
their mechanical properties 8 weeks in vitro at the level that ensures
their fixation properties. Pull-out tests indicated that the early version
of the studied antibiotic releasing miniscrew has lower values as compared
to controls (non-antibiotic containing SR-PLGA).
Antibiotic releasing SR-PLGA miniscrews inhibited bacteria on average
areas of ca. 30 mm in diameter when bacteria growth inhibition properties
were studied on agar plates. No inhibition was seen with control screws
(non-antibiotic containing bioabsorbable or titanium screws) (Fig.2).
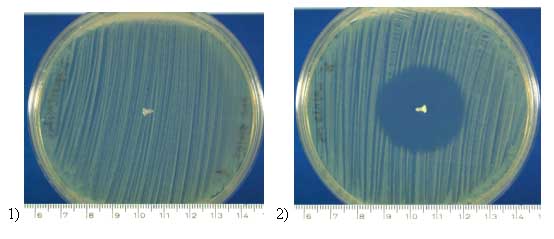
Fig.2. Ciprofloxacin releasing SR-PLGA miniscrew (2) inhibited bacteria
on average areas of ca. 30 mm in diameter. No inhibition was seen with
control screw (1).
Histology did not show much difference from the control plain SR-PLGA
screws except for some increased giant cells at some areas of the implantation
site.
Conclusions
Ciprofloxacin releasing SR-PLGA miniscrews are easy to install and
they have good drug release profiles, initial and in vitro mechanical
strengths,
bacterial inhibitory effect in vitro and biocompatibility. They may
be useful for clinical use in infection prophylaxis or therapy.
|

